One of the most exciting and intriguing findings in the subject of the evolution of oxygenic photosynthesis in the past few years is the discovery of the non-photosynthetic early-branching Cyanobacteria: the Vampirovibrionia (formerly Melainabacteria) (Soo et al. 2019) and the Sericytochromatia. They are considered to be within the phylum Cyanobacteria. The other really interesting new finding is the Margulisbacteria, which is considered to be the sister phylum to Cyanobacteria.
All the genomes of the known representatives of these novel clades are non-photosynthetic. Many of them live in environments where light cannot reach or live as symbionts. One of the very spectacular bugs in these new clades, for example, is Termititenax. This is a margulisbacterium that attaches to an ectosymbiotic spirochete of an oxymonad protist in the gut of termites (Utami et al. 2019). That is a three-level symbiosis involving four partners! Another interesting strain is Vampirovibrio chlorellavorus (Soo et al. 2015), which is a predatory bacterium that eats the eukaryote Chlorella!
It has been hypothesized that because these new clades do not have any photosynthetic representatives, the photosynthetic Cyanobacteria could have evolved photosynthesis at a relatively late stage, after the divergence of Vampirovibrionia.
I, and others, have said that the lineage from which the photosynthetic Cyanobacteria emerged is directly linked, through an unbroken line of descent, to the origin of photosynthesis itself. I have also said, based on the molecular evolution of the photosynthetic reaction centres, that the earliest stages in the evolution of photosynthesis predate the diversification of the major groups of bacteria. In other words, Margulisbacteria, Sericytochromatia, and Vampirovibrionia, as well as most, if not all bacteria, could have originated from photosynthetic ancestors. In a way very similar to methanogenesis in Archaea, which is thought to be the ancestral trait of the domain and was later lost repeatedly in many clades.
How do we prove however that an organism that today is the symbiont of a spirochete, which is the symbiont of an oxymonad, which is a symbiont of a termite, originated from a photosynthetic ancestor?
If the loss of a process, say a metabolic pathway, was relatively recent. Then, there may be traces of the lost pathway in the genome. Perhaps one of the enzymes in this lost pathway catalysed a type of reaction that could be used for something else and was therefore retained. Loss of photosynthesis in eukaryotes are common at most taxonomic levels, but it is much harder to prove it for bacteria. For example, the algae symbiont, Athelocyanobacterium thalassa, lost 75% of its genome in less than 100 million years (Cornejo-Castillo et al. 2016). Thus, very quickly, any trace of a photosynthetic past may have been erased after the ancestor of these clades became heterotrophic.
There are quite a few proteins in photosynthesis that can catalyse reactions that can be useful outside photosynthesis. So, there is a possibility that some of the free-living non-photosynthetic Cyanobacteria that retain larger genomes, from those lineages that did not become symbionts and/or experienced strong genome-size reductions, still contain remnants of photosynthesis proteins that have been co-opted for new functions.
Here I report two sequences that branch within the known diversity of bacteriochlorophyll a and chlorophyll a synthases, known as BchG and ChlG respective, in the metagenome-assembled genomes (MAGs) of two strains of Sericytochromatia. BchG and ChlG catalyse the final step in the synthesis of chlorophylls and bacteriochlorophylls and have the job of attaching the characteristic tails of these pigments to the tetrapyrrole ring.
I found one sequence using AnnoTree. There are four Sericytochromatia genomes and 53 Vampirovibronia genomes available there. I searched using the KEGG code K04040.
This one is found in the MAG GCA_002083825.1, contig DAZV01000039.1_72, which encodes 79 genes and it was published in Soo et al. (2017). The metagenome came from a coal bed methane well.
The second sequence I found using BLAST on the refseq database. It was the top hit when using the above sequence as query. This MAG was published in Parks et al. (2018) and the metagenome is from sediments. This is uncultivated Cyanobacteria bacterium UBA8530. This is the sequence and it was found in a contig encoding 12 genes.
I did not find BchG or ChlG in the Margulisbacteria MAGs available in AnnoTree.
The evolution of these synthases is very interesting because the enzyme is related to a key enzyme in Archaea, digeranylgeranylglyceryl phosphate synthase (DGGGPS), that is central to the discussions on the nature of the LUCA. I wrote about this a bit in a previous post: https://www.tanaicardona.com/blog/chlorophylls-the-origin-of-membranes-and-bioenergetics
These synthases are also related to other class of enzymes: for example, UbiA used in the synthesis of quinones or CyoE used in the synthesis of some types of heme.
The BLAST of these two sequences only retrieved (bacterio)chlorophyll synthases from phototrophs and suggested a bit of a phylogenetic distance. I collected some sequences and ran a Maximum Likelihood tree with PhyML.
Phylogeny of ChlG and BchG.
We can see in the figure above that the sequences from these strains branched together as sister to that from Heliobacteria. Although that position has no statistical support (0.1). Overall the level of sequence identity between the different BchG/ChlG sequences is in the region of just under 30%, but they align very well. At a sequence level they can be easily distinguished from other enzymes in the extended UbiA family due to the very large distances between the types.
Some Chlorobi and Chloroflexi have two BchG.
We can see that the sericytochromatian branches are the longest, more than twice as long as the standard synthases, suggesting faster rates of evolution.
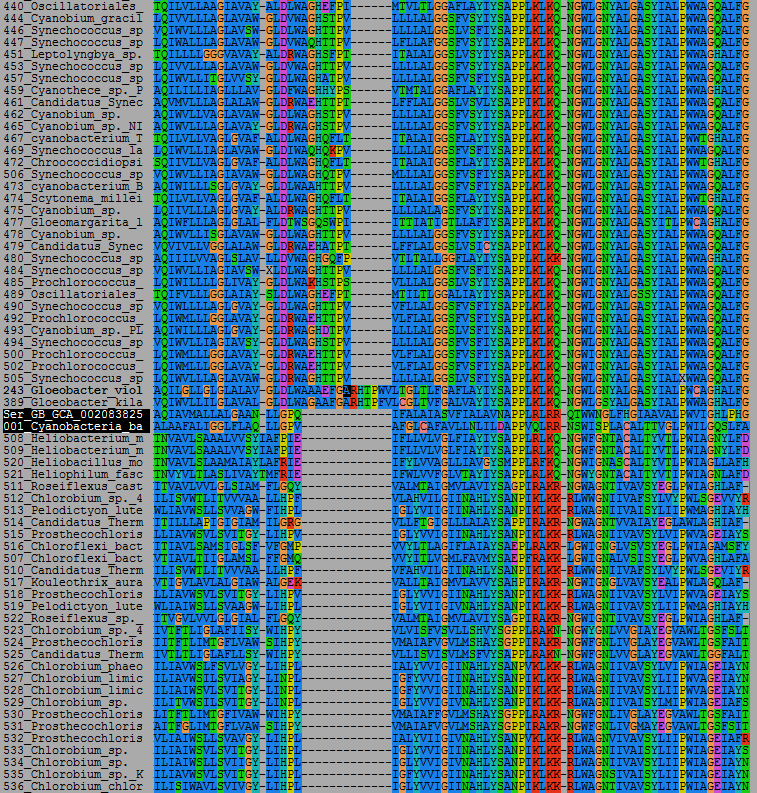
Unfortunately, we do not know a lot about the mechanism of function of these enzymes. There are no crystal structures and I was not able to find how chlorophylls bind or anything of the sort. Looking at the sequences, the sericytochromatian ones share a peculiar 13 amino acid gap with the BchG of the anoxygenic phototrophs, compared to a unique 13 amino acid insertion relative to the Gloeobacter sequences, and a 7 amino acid insertion compared to all other cyanobacterial sequences.
An alignment showing some interesting indels. This is just a part of the alignment. The sericytochromatian sequences are highlighted in black.
There is however no biochemical or structural evidence that could reveal what these differences mean in term of function. Overall, it appears as if they are more closely related to BchG than ChlG.
The large branches would be consistent with these sequences acquiring a novel function.
These synthases are membrane proteins, so I had a look at the secondary structure prediction and it turned out that overall the sericytochromatian sequences seem to be more like the cyanobacterial and chloroflexi sequences in topology, than those in other anoxygenic phototrophs, sharing 8 well-defined transmembrane helices.
Interestingly, unlike other UbiA-like proteins DGGGPS also has 8 predicted helices, which may therefore represent the ancestral state.
In any case, these are just superficial similarities and differences.
I had a look at the other genes encoded in the contigs and there was nothing that would stand out (to me) that could give some insight on the possible role of these proteins in the Sericytochromatia. In GCA_002083825.1, the sequence was two or three genes downstream of HemB, which is an enzyme in the synthesis pathway of porphyrins, but HemB was not in the UBA8530 contig. No other enzymes of the porphyrin synthesis pathway were found in the contigs.
Microbial mats from lake Vanda are known to contain a substantial number of free-living Vampirovibronia and Sericytochromatia strains. So, I BLASTed the sequence from GCA_002083825.1 against the Vanda metagenomes in the IMG/MER database, but I did not find any sequence that matched the Sericytochromatia ones, at least not within the complete sequences. I did get hits for Cyanobacteria and some of the other phototrophs as well as a eukaryotic alga.
It cannot be told whether these two sequences are ancestrally sericytochromatian or not, but these two strains, as well as their unique sequences, appear to be quite distant apart, so it may be an ancient legacy of a phototrophic past. They might just as well have been picked up from DNA of true phototrophs at some early point in time before they diverged and went on their separate pathways, as it is the case for genes encoding protochlorophyllide reductase found in two genomes of subsurface Altiarchaea: http://tanaiscience.blogspot.com/2017/05/a-new-undiscribed-clade-of-phototrophic.html
Given that there are very few Sericytochromatian MAGs available, only 9 in the Genome Taxonomy Database; and given that two of these had (B)ChlG, then it may be that this protein is somewhat widely distributed within this clade.
An apparently early-branching BchG was actually found in a contig from an archaeon and it was shown to have chlorophyll synthase activity (Meng et al. 2009). It would be interesting to do the same experiment with the sericytochromatian sequences! I might repeat the tree above at a later stage including this archaeal sequence, after doing a more extensive search of the metagenomes, and as I become more familiar with these type of enzymes and their other relatives.
I am compiling a number of candidate proteins that could be informative regarding the loss of photosynthesis in these clades for further study. Stay tuned for more.
References
Cornejo-Castillo, F. M., A. M. Cabello, G. Salazar, P. Sanchez-Baracaldo, G. Lima-Mendez, P. Hingamp, A. Alberti, S. Sunagawa, P. Bork, C. de Vargas, J. Raes, C. Bowler, P. Wincker, J. P. Zehr, J. M. Gasol, R. Massana and S. G. Acinas (2016). "Cyanobacterial symbionts diverged in the late Cretaceous towards lineage-specific nitrogen fixation factories in single-celled phytoplankton." Nat Commun 7. DOI: 10.1038/ncomms11071.
Meng, J., F. Wang, F. Wang, Y. Zheng, X. Peng, H. Zhou and X. Xiao (2009). "An uncultivated crenarchaeota contains functional bacteriochlorophyll a synthase." ISME J 3(1): 106-116. DOI: 10.1038/ismej.2008.85.
Parks, D. H., M. Chuvochina, D. W. Waite, C. Rinke, A. Skarshewski, P. A. Chaumeil and P. Hugenholtz (2018). "A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life." Nat Biotechnol 36(10): 996-1004 DOI: 10.1038/nbt.4229.
Soo, R. M., J. Hemp and P. Hugenholtz (2019). "Evolution of photosynthesis and aerobic respiration in the cyanobacteria." Free Radic Biol Med. DOI: 10.1016/j.freeradbiomed.2019.03.029.
Soo, R. M., J. Hemp, D. H. Parks, W. W. Fischer and P. Hugenholtz (2017). "On the origins of oxygenic photosynthesis and aerobic respiration in Cyanobacteria." Science 355(6332): 1436-1440. DOI: 10.1126/science.aal3794.
Soo, R. M., B. J. Woodcroft, D. H. Parks, G. W. Tyson and P. Hugenholtz (2015). "Back from the dead; the curious tale of the predatory cyanobacterium Vampirovibrio chlorellavorus." PeerJ 3: e968. DOI: 10.7717/peerj.968.
Utami, Y. D., H. Kuwahara, K. Igai, T. Murakami, K. Sugaya, T. Morikawa, Y. Nagura, M. Yuki, P. Deevong, T. Inoue, K. Kihara, N. Lo, A. Yamada, M. Ohkuma and Y. Hongoh (2019). "Genome analyses of uncultured TG2/ZB3 bacteria in 'Margulisbacteria' specifically attached to ectosymbiotic spirochetes of protists in the termite gut." ISME J 13(2): 455-467. DOI: 10.1038/s41396-018-0297-4.